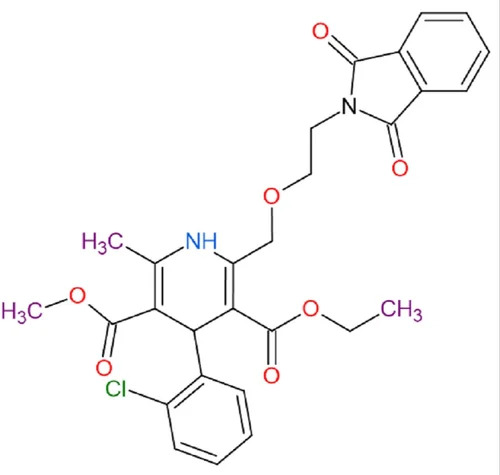
Phthaloyl Amlodipine
Product Details:
Phthaloyl Amlodipine Price And Quantity
- 2400 INR/Kilograms
- 100 Kilograms
- 105-108°C
- nan
- Soluble in DMSO and methanol
- 29420090
- Pharmaceutical Intermediates
- 2 years if stored properly
- Phthalimido derivative of Amlodipine
Phthaloyl Amlodipine Trade Information
- 10000 Kilograms Per Week
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
Phthaloyl Amlodipine is a derivative of Amlodipine which is a calcium channel blocker commonly used in the treatment of hypertension and angina The modification of Amlodipine through phthaloylation attachment of a phthaloyl group can potentially alter its pharmacokinetic properties or enhance its therapeutic effectiveness Below are the key details about this compound
Chemical Details
IUPAC Name 22Aminoethoxymethyl42ethoxyethoxyphthaloyl 14dihydro26dimethyl35pyridinecarboxylate
Molecular Formula C21H26N2O4
Molecular Weight 37045 gmol
Structure
Amlodipine a calcium channel blocker is modified by attaching a phthaloyl group derived from phthalic acid at one position
Physical Properties
Appearance Typically white to offwhite crystalline powder
Melting Point Likely higher than Amlodipine due to the presence of the phthaloyl group
Solubility Likely soluble in organic solvents like ethanol and methanol but less soluble in water
Applications
1 Pharmacological Use
Calcium Channel Blocker Like Amlodipine Phthaloyl Amlodipine would be expected to function as a calcium channel blocker affecting the smooth muscles in blood vessels leading to vasodilation and thus a reduction in blood pressure It may also be used to treat angina chest pain due to reduced blood flow to the heart
The phthaloyl group may enhance the lipophilicity allowing for improved membrane penetration or alter the drugs bioavailability or duration of action
2 Drug Development
Modifying Amlodipine with a phthaloyl group can be part of drug design efforts to optimize pharmacokinetics such as increasing solubility stability or the drugs affinity for specific receptors
Prodrugs In some cases introducing a phthaloyl group can be used to create a prodrug which would undergo enzymatic cleavage in the body to release the active Amlodipine molecule
3 Research
Preclinical Studies This compound could be of interest for preclinical pharmacological studies focused on understanding the role of phthaloylation in drug bioavailability receptor interactions or metabolism
Investigating the lipophilicity and membrane permeability changes imparted by the phthaloyl group could be key in optimizing its therapeutic profile
Chemical Reactivity
1 Phthaloyl Group C6H4CO2
The phthaloyl group is an aromatic ester and its attachment to Amlodipine can modify the compounds lipophilicity as phthaloyl groups are known to be less polar compared to the parent molecule
This modification could enhance the cell membrane permeability or biological halflife of the compound
2 Amlodipine Backbone
The Amlodipine structure consists of a dihydropyridine ring which is responsible for blocking calcium channels The phthaloyl modification could potentially influence the binding affinity to the calcium channel receptor or alter its pharmacokinetic profile
Synthesis of Phthaloyl Amlodipine
Step 1 Phthaloylation of Amlodipine React Amlodipine with phthalic anhydride or a phthaloyl chloride derivative in the presence of a suitable base eg pyridine triethylamine to form the phthaloyl derivative
Step 2 Purification and crystallization may follow to obtain the pure phthaloylated Amlodipine
Safety and Handling
Toxicity
The toxicity of Phthaloyl Amlodipine would be expected to follow that of Amlodipine but the phthaloyl group could potentially alter its safety profile Like other calcium channel blockers it may cause hypotension dizziness or peripheral edema
Precautions
Standard precautions for handling pharmaceutical intermediates should be followed including using gloves protective eyewear and working in a wellventilated area
Storage
Store in a tightly sealed container in a cool dry place away from light and sources of moisture
Potential Side Effects
Amlodipine Side Effects Since Phthaloyl Amlodipine is based on Amlodipine common side effects may include
Swelling in the lower legs or feet edema
Headaches
Dizziness or lightheadedness
Flushing
Nausea or abdominal pain
Conclusion
Phthaloyl Amlodipine may represent an interesting modification of Amlodipine aimed at optimizing its therapeutic properties The addition of a phthaloyl group could improve drug stability bioavailability or receptor interaction making it a potential candidate for further drug development or preclinical research Further studies would be needed to fully understand the pharmacokinetics and pharmacodynamics of this modified compound
Would you like more specific details on its synthesis potential applications or other derivatives of Amlodipine
Versatile Solubility for Formulation Needs
Phthaloyl Amlodipines solubility in both DMSO and methanol ensures compatibility with a wide range of laboratory and industrial solutions. This feature makes it highly practical for synthetic processes and product formulation in pharmaceutical research and development.
Stable and Reliable Shelf Life
With a shelf life of two years when properly stored, Phthaloyl Amlodipine remains a reliable option for long-term use. Pharmaceutical companies can manage inventory confidently, knowing the compound maintains its integrity over time.
FAQs of Phthaloyl Amlodipine:
Q: How should Phthaloyl Amlodipine be stored to maximize its shelf life?
A: For optimal shelf life of two years, Phthaloyl Amlodipine should be stored in a cool, dry place away from direct sunlight and moisture, following standard pharmaceutical intermediate storage protocols.Q: What are the common solvents for dissolving Phthaloyl Amlodipine?
A: Phthaloyl Amlodipine is readily soluble in DMSO (dimethyl sulfoxide) and methanol, making it suitable for various laboratory and industrial applications requiring these solvents.Q: When is it beneficial to use Phthaloyl Amlodipine in pharmaceutical synthesis?
A: This compound is especially beneficial during the intermediate stages of pharmaceutical synthesis, aiding the production of active pharmaceutical ingredients due to its stable properties and suitable melting range.Q: Where can Phthaloyl Amlodipine be sourced in India?
A: Phthaloyl Amlodipine is supplied by distributors, manufacturers, traders, and suppliers across India, often listed under HS Code 29420090 for pharmaceutical intermediates.Q: What is the manufacturing process involvement for Phthaloyl Amlodipine?
A: Phthaloyl Amlodipine is synthesized as the phthalimido derivative of Amlodipine, typically produced via controlled organic reactions adhering to pharmaceutical standards.Q: How is Phthaloyl Amlodipine used by pharmaceutical companies?
A: Pharmaceutical companies use this compound as an intermediate to develop or modify active ingredients, leveraging its chemical properties for targeted synthesis.Q: What key benefits does Phthaloyl Amlodipine offer to pharmaceutical manufacturers?
A: Its moderate melting point, efficient solubility, and dependable shelf life enhance formulation flexibility and supply chain reliability for manufacturers in the pharmaceutical sector.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+