
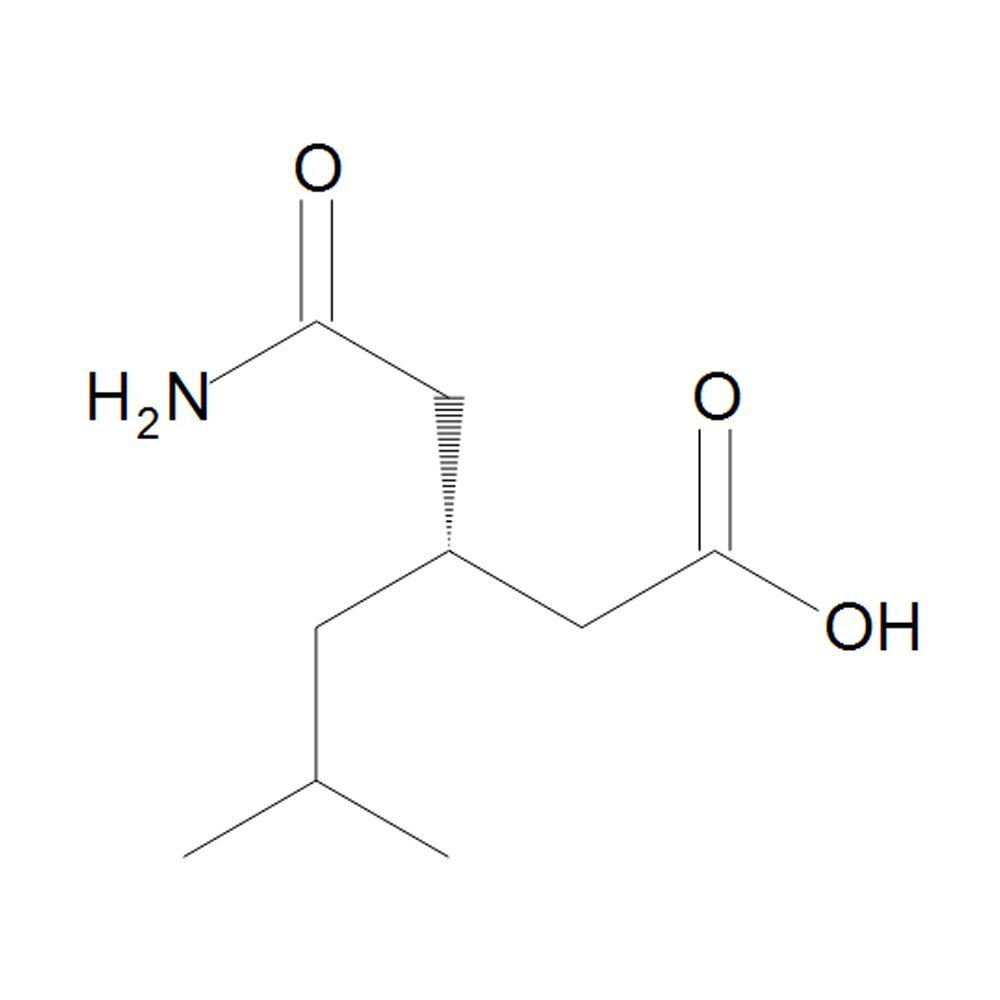
(R)-(-)-3-(Carbamoylmethyl)- 5-methyl hexanoic acid (RCMH)
Product Details:
(R)-(-)-3-(Carbamoylmethyl)- 5-methyl hexanoic acid (RCMH) Price And Quantity
- 100 Kilograms
- 1400 INR/Kilograms
(R)-(-)-3-(Carbamoylmethyl)- 5-methyl hexanoic acid (RCMH) Trade Information
- Others
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
R3Carbamoylmethyl5methyl hexanoic acid RCMH is the enantiomerically pure Rform of the compound indicating it is optically active and contains only the Rconfiguration at its chiral center This compound is of interest in medicinal chemistry particularly for its potential role in modulating neurological processes
Structural and Chemical Details
Chemical formula C10H19NO3
Molecular weight 20126 gmol
Chirality The Rconfiguration specifies the spatial arrangement of the groups around the chiral center
Functional Groups
Carboxylic Acid COOH Imparts acidity and potential for forming salts or esters
Carbamoylmethyl Group CH2CONH2 Contributes to hydrogen bonding and potential interactions with biological targets
Methyl Substituent Located on the hexanoic acid backbone influencing hydrophobicity and steric interactions
Pharmaceutical Context
1 Neurological Activity
Similar compounds eg pregabalin act as GABA analogs modulating calcium channels 2 subunit to treat neuropathic pain epilepsy and anxiety The Renantiomer is often the active form in such cases due to stereospecific receptor interactions
2 Enantiomeric Purity
The pharmacological activity and metabolism of chiral compounds can be heavily influenced by their configuration The Renantiomer may exhibit distinct bioactivity compared to the racemic mixture or the Sform
Potential Applications
Therapeutic Research The compound may be explored for its role in
Neuropathic pain management
Seizure modulation
Anxiety treatment
Drug Development Used as a lead compound or intermediate for synthesizing derivatives with improved potency bioavailability or safety
Synthesis
The synthesis of R3Carbamoylmethyl5methyl hexanoic acid typically involves
1 Chiral Resolution Separating the R and Senantiomers from a racemic mixture
2 Asymmetric Synthesis Employing chiral catalysts or reagents to selectively produce the Renantiomer
Let me know if you need more details on synthesis biological activity or a specific application for this compound

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



 Call Me Free
Call Me Free
