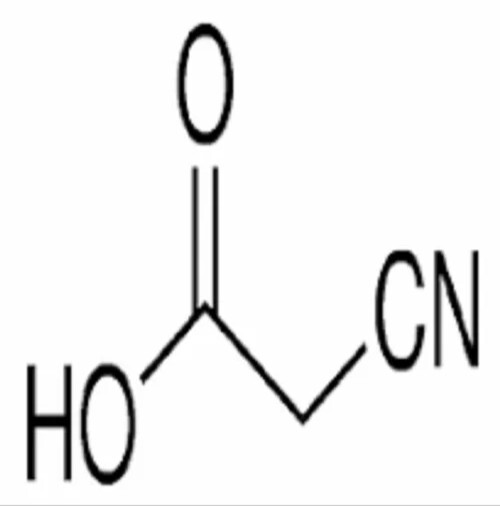
Cynoacetic acid
Product Details:
Cynoacetic acid Price And Quantity
- 110 INR/Kilograms
- 1000 Kilograms
- 372-09-8
- 2 Years
- Solid
- Cynoacetic Acid
- 99% min
- Not determined
- 2926
- Not determined
- 85.06 g/mol
- Keep container tightly closed in a dry and well-ventilated place
- C3H3NO2
- IRRITANT
- 63-65C
- Bags/Drums
- 206-759-6
- Cyanoacetic acid, -Cyanoacetic acid
- Odorless
- Soluble in water, alcohol, and acetone
- White crystalline powder
- Intermediate for pharmaceuticals, agrochemicals, and organic synthesis
Cynoacetic acid Trade Information
- Others
- 1000 Kilograms Per Week
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
It seems you are referring to cyanoacetic acid a compound used extensively in organic synthesis Heres an overview
Chemical Details
IUPAC Name Cyanoacetic acid
Molecular Formula C3H3NO2
Molecular Weight 8506 gmol
Structure
Contains a carboxylic acid group COOH and a nitrile group CN attached to the same carbon atom
Physical Properties
Appearance White crystalline solid
Melting Point 7580C
Boiling Point Decomposes before boiling
Solubility Soluble in water alcohol and other polar organic solvents
Chemical Characteristics
1 Acidity
Cyanoacetic acid is a stronger acid compared to simple carboxylic acids due to the electronwithdrawing effect of the nitrile group
pKa 23
2 Reactivity
Both functional groups carboxylic acid and nitrile make it a versatile reagent in organic chemistry
The methylene group CH2 adjacent to these electronwithdrawing groups is highly reactive and acidic enabling enolate formation
Applications
1 Organic Synthesis
Malonic Acid Derivative Serves as a precursor in the synthesis of malonic acid derivatives
Intermediate for Pharmaceuticals Used in synthesizing barbiturates nonsteroidal antiinflammatory drugs NSAIDs and other active pharmaceutical ingredients APIs
Heterocyclic Compounds Key starting material for pyrimidines indoles and other heterocycles
2 Industrial Use
In the production of dyes agricultural chemicals like herbicides and insecticides and adhesives
3 Michael Donor
Due to its activated methylene group cyanoacetic acid participates in Michael addition reactions forming carboncarbon bonds
Synthesis of Cyanoacetic Acid
Typically produced by the reaction of chloroacetic acid with sodium cyanide
textClCH_2textCOOH textNaCN rightarrow textNCCH_2textCOOH textNaCl
Safety and Handling
Toxicity
Harmful if ingested or inhaled Irritates skin eyes and respiratory tract
Handle with care in a fume hood with appropriate protective gear
Storage
Store in a cool dry place away from strong oxidizing or reducing agents
Would you like to explore specific reaction pathways industrial processes or applications of cyanoacetic acid in greater depth
Key Applications in Synthesis
Cynoacetic acid stands out as a crucial intermediate in the synthesis of medicines, agrochemicals, and a variety of fine chemicals. Its versatile reactivity allows for the production of compounds essential in healthcare and crop science industries, making it indispensable for chemical manufacturers. With a high purity grade of 99%, this compound ensures reliable performance in industrial processes.
Physical Properties and Packaging
Appearing as a white crystalline powder and remaining odorless, Cynoacetic acid is soluble in water, alcohol, and acetone, making it easy to handle in most laboratory and industrial settings. It is supplied in bags or drums to maintain product integrity during storage and transport. For optimal preservation, containers should be tightly closed and stored in dry, well-ventilated spaces.
FAQ's of Cynoacetic acid:
Q: How should Cynoacetic acid be stored to maintain its quality?
A: Cynoacetic acid should be kept in tightly closed containers placed in a dry, well-ventilated area. Proper storage helps preserve its 99% purity and extends its shelf life to 2 years.Q: What are the main industrial applications of Cynoacetic acid?
A: Cynoacetic acid is primarily used as an intermediate in the manufacture of pharmaceuticals, agrochemicals, and for a variety of organic synthesis processes.Q: When is Cynoacetic acid typically utilized in synthesis processes?
A: It is employed during the intermediate stages of chemical manufacturing, allowing for the preparation of more complex compounds required in drug and agrochemical production.Q: Where is Cynoacetic acid commonly supplied and distributed?
A: Cynoacetic acid is distributed, manufactured, supplied, and traded widely in India, meeting the needs of both research and industry sectors.Q: What is the process for safely handling Cynoacetic acid?
A: Due to its classification as an irritant, appropriate personal protective equipment such as gloves and goggles should be worn when handling Cynoacetic acid. Work in a well-ventilated area and avoid contact with eyes and skin.Q: What are the benefits of using Cynoacetic acid in synthesis?
A: The main benefits include its high purity, versatility in various organic syntheses, and high solubility in common solvents, all of which contribute to efficient and reliable manufacturing outcomes.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



![Dibenzo-[B,F][1,4]-Thiazepin-11(10H)- one](https://cpimg.tistatic.com/10259416/b/4/Dibenzo-B-F-1-4-Thiazepin-11-10H-one.jpg?tr=w300)