
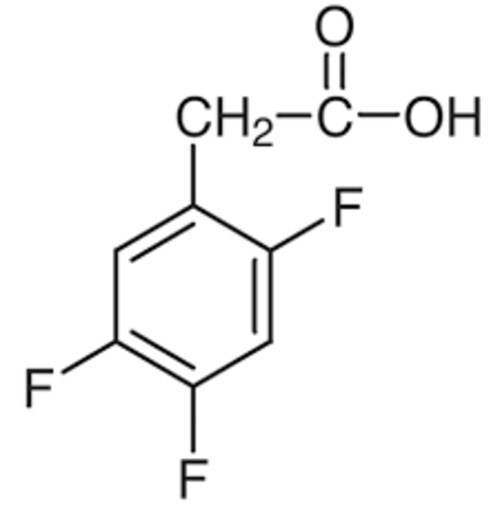
2,4,5 Trifluro phenyl acetic acid
Product Details:
2,4,5 Trifluro phenyl acetic acid Price And Quantity
- 3300 INR/Kilograms
- 100 Kilograms
2,4,5 Trifluro phenyl acetic acid Trade Information
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
245Trifluorophenylacetic acid is a chemical compound that contains a phenylacetic acid backbone with three fluorine atoms attached at the 2 4 and 5 positions of the phenyl ring This compound is of interest in various fields including medicinal chemistry agrochemical development and organic synthesis owing to the influence of the trifluoromethyl group on its chemical and biological properties
Chemical Details
IUPAC Name 245Trifluorophenylacetic acid
Molecular Formula C8H6F3CO2
Molecular Weight 22013 gmol
Structure
The compound consists of a phenyl ring C6H5 substituted with three fluorine atoms at the 2 4 and 5positions The phenyl ring is attached to an acetic acid group COOH via the 1position of the phenyl ring
Physical Properties
Appearance Typically a white to offwhite crystalline solid
Melting Point 118122C depending on purity
Boiling Point Data not widely available but expected to be high due to the phenyl group and carboxylic acid
Solubility Soluble in polar organic solvents like methanol ethanol and acetone Poorly soluble in water
Density 146 gcm
Applications
1 Medicinal Chemistry
Fluorinated Aromatic Compounds Trifluorophenylacetic acid and its derivatives are studied in medicinal chemistry for their potential bioactivity The introduction of fluorine atoms can increase lipophilicity stability and the ability of the compound to penetrate cell membranes making it useful for drug design
Nonsteroidal Antiinflammatory Drugs NSAIDs Some fluorinated acetic acids are used in the development of NSAIDs which are designed to reduce inflammation and pain The trifluoromethyl group is known to enhance the potency and specificity of these compounds
Potential Anticancer and Antibacterial Properties Fluorinated phenylacetic acid derivatives can also be evaluated for anticancer and antibacterial activity due to their structural similarity to other bioactive compounds
2 Agrochemicals
Herbicide Development Compounds with fluorine atoms especially those containing trifluoromethyl groups are often studied for their potential as herbicides or pesticides Fluorine atoms can alter the herbicidal properties of phenylacetic acids improving their selectivity and effectiveness
Plant Growth Regulators The trifluorophenyl group can modify the behavior of plant hormones or other biologically active molecules potentially leading to the development of growth regulators or defoliants
3 Organic Synthesis
Intermediate in Chemical Synthesis Trifluorophenylacetic acid is used as an intermediate in the synthesis of complex organic molecules including pharmaceuticals and agrochemicals The trifluoromethyl group can facilitate certain reactions such as electrophilic substitution and nucleophilic aromatic substitution
Building Block for Fluorinecontaining Compounds This compound is useful as a starting material in the preparation of other fluorinated aromatic compounds where the trifluoromethyl group is a key feature
Chemical Reactivity
1 Electrophilic Aromatic Substitution
The trifluoromethyl group is an electronwithdrawing group which can deactivate the aromatic ring towards electrophilic substitution reactions at the 3 and 6positions relative to the carboxylic acid group This makes it possible to selectively functionalize the compound
2 Nucleophilic Aromatic Substitution
The trifluoromethyl group can make the aromatic ring more reactive in nucleophilic aromatic substitution reactions particularly under basic conditions or with strong nucleophiles
3 Decarboxylation Reactions
The carboxylic acid group COOH could undergo decarboxylation reactions under high temperature or basic conditions potentially leading to the formation of fluorinated aromatic hydrocarbons
4 Reduction Reactions
The carboxylic acid group can be reduced to an alcohol group creating trifluorophenylethanol which may be useful for further reactions
Synthesis of 245Trifluorophenylacetic Acid
1 Fluorination of Phenylacetic Acid
Fluorine gas or fluorinating reagents such as Selectfluor can be used to introduce the trifluoromethyl group at the 2 4 and 5positions of the phenyl ring
Electrophilic Aromatic Substitution Phenylacetic acid can be fluorinated under controlled conditions to selectively introduce fluorine atoms at the desired positions Catalysts such as silver fluoride AgF or potassium fluoride KF may be used to assist in the reaction
2 Alternative Synthetic Routes
The compound can be synthesized from commercially available 245trifluorobenzyl chloride by nucleophilic substitution with sodium acetate NaOAc or another acetate source under appropriate conditions to form the acetic acid derivative
Safety and Handling
Toxicity
As with most fluorinated aromatic compounds 245trifluorophenylacetic acid should be handled with care It may irritate the eyes skin or respiratory system Prolonged exposure could have adverse effects
Precautions
Wear appropriate PPE gloves safety goggles lab coat when handling the compound
Work in a wellventilated area or fume hood to avoid inhalation of vapors or dust
Avoid ingestion and direct contact with the skin
Storage
Store in a cool dry place in tightly sealed containers Avoid exposure to moisture as fluorinated compounds can be reactive under certain conditions
Conclusion
245Trifluorophenylacetic acid is a versatile fluorinated compound with potential applications in medicinal chemistry agrochemicals and organic synthesis The trifluoromethyl group significantly influences the chemical reactivity and biological activity of this compound making it a valuable building block for further research and development in these fields
Would you like additional details on its applications in specific areas or potential modifications to its structure

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



 Call Me Free
Call Me Free
