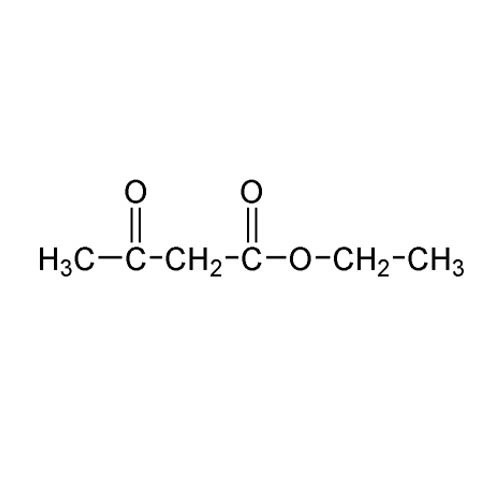
Ethyl 4-(2- Phthalimidoethoxy) acetoacetate(PHEEMA)
Product Details:
Ethyl 4-(2- Phthalimidoethoxy) acetoacetate(PHEEMA) Price And Quantity
- 100 Kilograms
- 1700 INR/Kilograms
Ethyl 4-(2- Phthalimidoethoxy) acetoacetate(PHEEMA) Trade Information
- Others
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
Ethyl 42Phthalimidoethoxyacetoacetate abbreviated as PHEEMA is a chemical compound that incorporates a phthalimido group and an acetoacetate ester functional group It is typically used in organic synthesis and medicinal chemistry potentially in the development of novel bioactive compounds or as a reagent in chemical transformations
Chemical Details
IUPAC Name Ethyl 42Phthalimidoethoxyacetoacetate
Molecular Formula C14H15NO4
Molecular Weight 26128 gmol
Structure
The molecule contains an ethyl ester group at the acetoacetate position which is a keto ester and a phthalimidoethoxy group at the 4position of the acetoacetate ester
Physical Properties
Appearance White to offwhite crystalline solid or powder
Melting Point Typically between 100120C though it can vary based on purity
Boiling Point Not readily available but would be high due to the ester and phthalimide groups
Solubility Soluble in common organic solvents like ethanol acetone and chloroform but likely poorly soluble in water due to the hydrophobic nature of the phthalimide and ethoxy groups
Density 128 gcm
Applications
1 Organic Synthesis
Building Block for Drug Synthesis PHEEMA can serve as an intermediate in the synthesis of phthalimide derivatives and other biologically active molecules
Key Reagent for Reactions It can participate in nucleophilic substitution reactions where the phthalimido group can be modified and in esterification reactions to form novel derivatives with potential applications in medicinal chemistry
2 Medicinal Chemistry
Phthalimide Derivatives Phthalimide derivatives are known for their potential neuroactive anticonvulsant and antitumor activities The functionalization of acetoacetate with a phthalimido group might result in compounds with useful biological properties
Prodrugs PHEEMA could be explored as a prodrug or a precursor to compounds that exhibit enhanced bioavailability or specific pharmacological properties
3 Biological Research
The ethoxy group in combination with the phthalimido group could influence the compounds ability to cross cellular membranes making it a candidate for cellular penetration studies or drug delivery systems
4 Potential Applications in Agrochemicals
Similar to other phthalimide derivatives PHEEMA could be evaluated as a pesticide or fungicide precursor due to its ability to interact with biological systems though its primary use is more likely in medicinal chemistry
Chemical Reactivity
1 Nucleophilic Substitution
The ethoxy group in the structure might undergo nucleophilic substitution reactions with various nucleophiles allowing for the synthesis of other derivatives or functionalized compounds
2 Enolization Acetoacetate Group
The acetoacetate group is reactive in enolization reactions where it forms an enolate ion making it useful in Michael addition reactions aldol condensations or Knoevenagel condensations to form carboncarbon bonds
3 Reduction Reactions
The ester group can be reduced to a hydroxyester which could potentially lead to the formation of alcoholcontaining compounds useful in further synthetic steps
Synthesis of Ethyl 42Phthalimidoethoxyacetoacetate PHEEMA
1 Step 1 Synthesis of 2Phthalimidoethanol
A phthalic anhydride or phthalimide can react with ethanolamine or ethanol to form the phthalimidoethoxy group resulting in the intermediate 2phthalimidoethanol
2 Step 2 Esterification
The 2phthalimidoethanol is then esterified with ethyl acetoacetate using a base like pyridine or sodium ethoxide to form PHEEMA
Safety and Handling
Toxicity
PHEEMA and similar phthalimide derivatives should be handled with care The phthalimide group can be toxic and may irritate the skin eyes or respiratory system upon exposure
Precautions
Wear appropriate personal protective equipment PPE such as gloves goggles and work under a fume hood to avoid inhalation or contact with the skin
Storage
Store in a cool dry place in tightly sealed containers away from heat and moisture to maintain stability
Conclusion
Ethyl 42Phthalimidoethoxyacetoacetate PHEEMA is a versatile compound with potential applications in medicinal chemistry particularly as a precursor to phthalimide derivatives and other bioactive molecules Its structure combining a phthalimido group with an acetoacetate ester makes it a useful building block for organic synthesis with possible therapeutic implications such as anticonvulsant or antitumor activity
Would you like additional information on its derivatives specific synthetic methods or potential applications in drug development

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+