
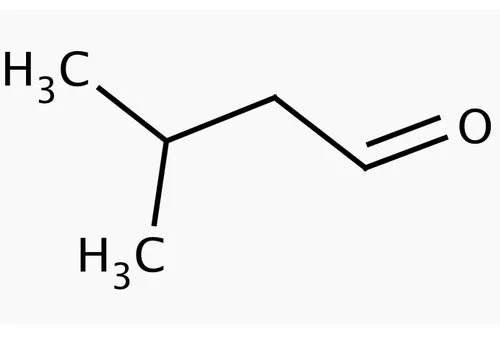
Isovaleraldehyde 590-86-3
Product Details:
Isovaleraldehyde 590-86-3 Price And Quantity
- 265 INR/Kilograms
- 1000 Kilograms
Isovaleraldehyde 590-86-3 Trade Information
- 1000 Kilograms Per Week
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
Isovaleraldehyde is an organic compound classified as an aldehyde It is notable for its role as a flavor and fragrance compound and as an intermediate in organic synthesis Below is a detailed overview
Chemical Details
IUPAC Name 3Methylbutanal
Molecular Formula C5H10O
Molecular Weight 8613 gmol
Structure
A fivecarbon chain with a terminal aldehyde group CHO and a methyl group attached to the third carbon
Physical Properties
Appearance Clear colorless to pale yellow liquid
Odor Pungent fruity characteristic of branched aldehydes
Boiling Point 92C
Density 079 gcm at 20C
Applications
1 Flavor and Fragrance Industry
Used to impart a fruity or malty aroma to various products
Found naturally in some fermented beverages and foods
Commonly utilized in artificial flavoring for baked goods candies and alcoholic beverages
2 Organic Synthesis
Intermediate Used to synthesize other compounds such as isovaleric acid amines or esters
Building Block Serves as a precursor in the synthesis of pharmaceuticals and agrochemicals
3 Biochemical Role
A byproduct of certain metabolic pathways especially during amino acid catabolism eg leucine
Industrial Relevance
Production
Typically produced through the oxidation of isovaleryl alcohol or by hydroformylation of isobutene
Key Derivatives
Isovaleric Acid A carboxylic acid with wide industrial use
Esters Used in perfumes and flavorings due to their pleasant odors
Safety and Handling
Toxicity
Can cause irritation to the eyes skin and respiratory tract
Prolonged exposure to high concentrations may have toxic effects
Precautions
Store in a cool wellventilated area away from oxidizing agents
Use personal protective equipment gloves goggles when handling
Chemical Reactions
1 Oxidation
Isovaleraldehyde can be oxidized to isovaleric acid using mild oxidizing agents like potassium permanganate or chromic acid
2 Condensation Reactions
The aldehyde group allows it to participate in aldol condensation or similar reactions forming larger organic molecules
3 Reduction
Can be reduced to isovaleryl alcohol using reducing agents like sodium borohydride NaBH4 or lithium aluminum hydride LiAlH4
Would you like details on its synthesis natural occurrence or specific industrial applications

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



![Dibenzo-[B,F][1,4]-Thiazepin-11(10H)- one](https://cpimg.tistatic.com/10259416/b/4/Dibenzo-B-F-1-4-Thiazepin-11-10H-one.jpg?tr=w300)
 Call Me Free
Call Me Free
