
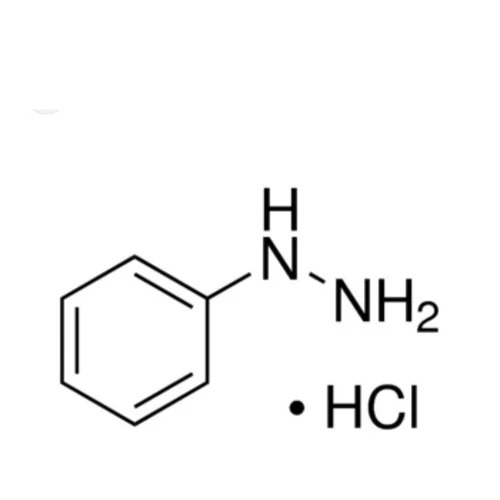
4-Sulfonamide Phenylhydrazine Hydrochloride
Product Details:
4-Sulfonamide Phenylhydrazine Hydrochloride Price And Quantity
- 450 INR/Kilograms
- 1000 Kilograms
4-Sulfonamide Phenylhydrazine Hydrochloride Trade Information
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
4Sulfonamide Phenylhydrazine Hydrochloride
Chemical Overview
Molecular Formula CHClNOS
Molecular Weight 20867 gmol
Functional Groups
Sulfonamide SONH
Phenylhydrazine NHNH
Hydrochloride salt HCl
Structure Properties
This compound consists of
1 Phenylhydrazine core NHNH on a benzene ring known for its reactivity with carbonyl compounds
2 Sulfonamide SONH at the para 4 position confers antibacterial and bioactive properties
3 Hydrochloride salt HCl form increases water solubility and stability
Applications
1 Pharmaceutical Medicinal Chemistry
Potential antimicrobial agent due to the sulfonamide group
May act as a precursor or intermediate in drug synthesis
Related to phenylhydrazine derivatives which interact with ketones and aldehydes
2 Analytical Chemistry
Could be used in derivatization reactions for detecting carbonylcontaining compounds
3 Biochemical Research
Phenylhydrazine derivatives have been studied for their effects on oxidative stress and enzyme inhibition
Reactivity Safety
Reactivity
Can form hydrazones with aldehydesketones
Sulfonamide group can participate in hydrogen bonding affecting drugreceptor interactions
Toxicity Handling
Phenylhydrazines are known to be toxic and potentially mutagenic
Handle with gloves eye protection and a fume hood

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+





 Call Me Free
Call Me Free
