
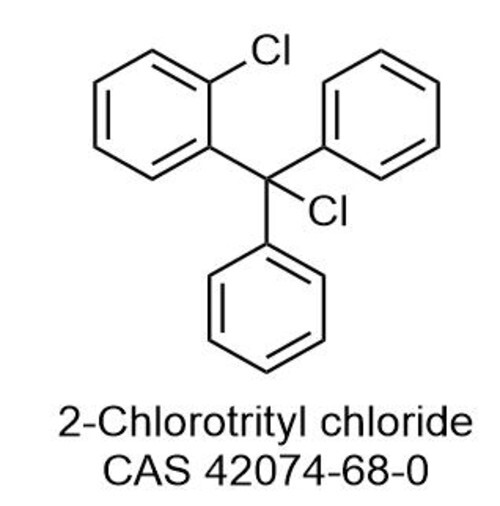
2-Chlorotriyl Choride (2CTC)
Product Details:
2-Chlorotriyl Choride (2CTC) Price And Quantity
- 1300 INR/Kilograms
- 1000 Kilograms
2-Chlorotriyl Choride (2CTC) Trade Information
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
2Chlorotriyl chloride often abbreviated as 2CTC is a chemical compound that contains a chlorotriyl group typically used in organic synthesis and as a reagent in various chemical processes
Chemical Details
IUPAC Name 2Chlorotriyl chloride
Molecular Formula C6H3Cl2
Molecular Weight 17100 gmol
Structure
It consists of a benzene ring C6H5 with two chlorine atoms attached at the 2 and 1positions on the aromatic ring
Physical Properties
Appearance Typically colorless to pale yellow liquid or solid depending on the state
Boiling Point 239242C
Melting Point Approximately 8082C solid
Solubility Soluble in organic solvents like acetone ethanol and chloroform but poorly soluble in water due to the presence of the chlorine atoms
Density Around 145 gcm at room temperature
Applications
1 Organic Synthesis
Chlorinating Agent The chlorine in 2chlorotriyl chloride can serve as a chlorinating agent in organic reactions facilitating the introduction of chlorine into other molecules
Building Block for Syntheses It can be used as a precursor in the synthesis of various organic compounds or as a reagent for introducing the chlorotriyl group into a target molecule
2 Polymer Chemistry
Used in the synthesis of chlorinecontaining polymers The reactivity of the chlorine can lead to the formation of complex polymeric structures
3 Agrochemical Synthesis
It could be used as an intermediate in the preparation of pesticides or other agrochemical products that require chlorinated aromatic compounds
4 Pharmaceutical Chemistry
Potential applications in pharmaceutical research where a chlorinated aromatic compound is required such as in the development of antibiotics or other biologically active compounds
Chemical Reactivity
1 Electrophilic Substitution
The presence of chlorine in the 2position makes the compound reactive in electrophilic aromatic substitution reactions Chlorine is an electronwithdrawing group which can activate the aromatic ring for further reactions
2 Nucleophilic Substitution
The chlorine atom at position 2 can be replaced by nucleophiles in substitution reactions such as with amines alcohols or thiols creating a variety of substituted products
3 CrossCoupling Reactions
Chlorotriyl chloride can participate in crosscoupling reactions such as Suzuki coupling or Negishi coupling allowing the formation of more complex organic structures
Synthesis of 2Chlorotriyl Chloride
The synthesis of 2chlorotriyl chloride can typically be done via halogenation reactions
Chlorination of Triyl Compounds The chlorination of triaryl compounds such as biphenyl or toluene derivatives can produce various halogenated species including 2chlorotriyl chloride Chlorine gas or phosphorus trichloride PCl is often used as a chlorinating agent
Safety and Handling
Toxicity
2Chlorotriyl chloride and its derivatives may be toxic if ingested or inhaled and should be handled with care It may cause irritation to the eyes skin and respiratory tract
Precautions
Wear appropriate personal protective equipment PPE including gloves safety goggles and work under a fume hood to avoid inhalation
Avoid exposure to the substance as it may be harmful or irritating in the long term
Storage
Store in a cool dry place away from heat moisture or sources of ignition The container should be tightly sealed to avoid contamination or accidental release
Conclusion
2Chlorotriyl chloride 2CTC is an important intermediate in organic chemistry particularly for reactions involving chlorination and electrophilic substitution Its utility extends across various industries including pharmaceuticals agrochemicals and materials science As a chlorinated aromatic compound it serves as a key building block for more complex chemical syntheses
Would you like additional information on its applications synthetic routes or related compounds

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+





 Call Me Free
Call Me Free
