
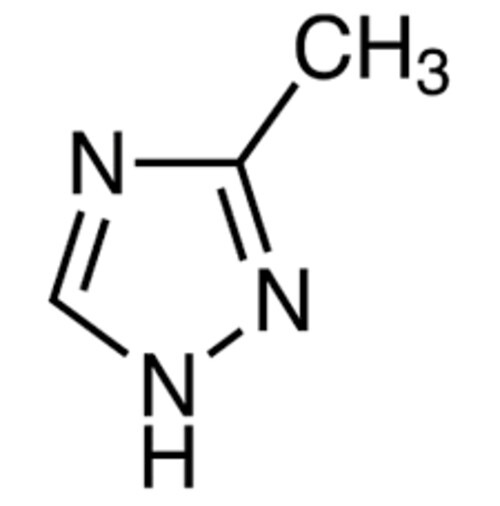
1H-1,2,4- triazole
Product Details:
1H-1,2,4- triazole Price And Quantity
- 1000 Kilograms
- 450 INR/Kilograms
1H-1,2,4- triazole Trade Information
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
1H124Triazole is a heterocyclic compound containing a fivemembered ring made up of three nitrogen atoms and two carbon atoms with a hydrogen atom attached to one of the nitrogen atoms in the ring It is one of the most common triazole derivatives and has significant importance in chemistry and various fields such as pharmaceuticals and material science
Chemical Details
IUPAC Name 1H124Triazole
Molecular Formula C3H3N3
Molecular Weight 6907 gmol
Structure
A triazole ring with nitrogen atoms at positions 1 2 and 4 The H in 1H refers to the hydrogen atom at the 1position
Physical Properties
Appearance Colorless to pale yellow crystalline solid
Melting Point 7072C
Boiling Point 175C
Solubility Soluble in water and polar organic solvents like ethanol and acetone
Density 125 gcm
Applications
1 Pharmaceuticals
Antifungal Activity 1H124triazole derivatives are widely used as antifungal agents For example fluconazole itraconazole and voriconazole are derived from the triazole ring structure and are used to treat various fungal infections by inhibiting ergosterol biosynthesis
Anticancer Potential Triazole derivatives are also explored for their anticancer properties particularly in inhibiting enzymes involved in DNA synthesis and repair
Antiinflammatory and Antibacterial Activity Some derivatives show promise in antiinflammatory and antibacterial applications
2 Agriculture
Fungicides Triazole compounds are used as fungicides in agriculture due to their ability to inhibit the biosynthesis of ergosterol which is crucial for fungal cell membranes
Herbicides and Growth Regulators Some triazole derivatives are used to modify plant growth or as plant growth regulators in crops
3 Materials Science
Coordination Chemistry The nitrogen atoms in the triazole ring can coordinate with metal centers making 1H124triazole useful in the synthesis of metalorganic frameworks MOFs or other coordination compounds
Polymer Chemistry Triazole derivatives are used in the synthesis of specialized polymers often to impart unique properties like resistance to oxidation or enhanced mechanical strength
4 Chemical Synthesis
Ligands in Organic Synthesis The triazole ring acts as a bidentate ligand for metals widely used in catalysis
Reagents in Organic Synthesis 1H124Triazole is used as a building block in the synthesis of other functionalized heterocycles or complex organic molecules
Chemical Reactivity
1 Nucleophilic Substitution
The nitrogen atoms in the 124triazole ring can act as nucleophiles enabling substitution reactions in organic synthesis
2 Electrophilic Aromatic Substitution
The triazole ring is activated at the positions 3 and 5 relative to the nitrogen atoms making it reactive in electrophilic substitution reactions
3 Coordination Chemistry
The nitrogen atoms in the triazole ring can coordinate with metal centers forming stable chelates useful in catalytic processes or material science
Synthesis of 1H124Triazole
1 From Hydrazine Derivatives
One common synthetic route involves the reaction of hydrazine with an unsaturated ketone or other appropriate reagents to form the triazole ring
2 Via Cyclization of Azides
Triazoles can be synthesized by cyclization of azides with alkynes or other electrophilic reagents providing a straightforward method for constructing the ring
3 From Amidrazones or Hydrazones
Cyclization of amidrazones or hydrazones with appropriate electrophiles or acid catalysts leads to 1H124triazole
Safety and Handling
Toxicity
1H124Triazole and its derivatives should be handled with care as they may be harmful if ingested inhaled or absorbed through the skin
It may cause irritation to the skin eyes and respiratory system
Precautions
Use gloves protective eyewear and work in a wellventilated fume hood Always follow safety guidelines for handling chemicals
Storage
Store in a cool dry place away from heat moisture and strong oxidizers Ensure containers are tightly sealed
Would you like additional information on specific derivatives of 1H124triazole its role in drug design or its use in coordination chemistry

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+





 Call Me Free
Call Me Free
